Identifying urine biomarkers that are reflective of renal pathology changes in lupus nephritis
Lead: Ting Zhang
Team members: Hao Li, Kamala Vanarsa, Gabriel Gidley
Collaborator: Drs. Petri, Saxena, Mok
Project Summary:
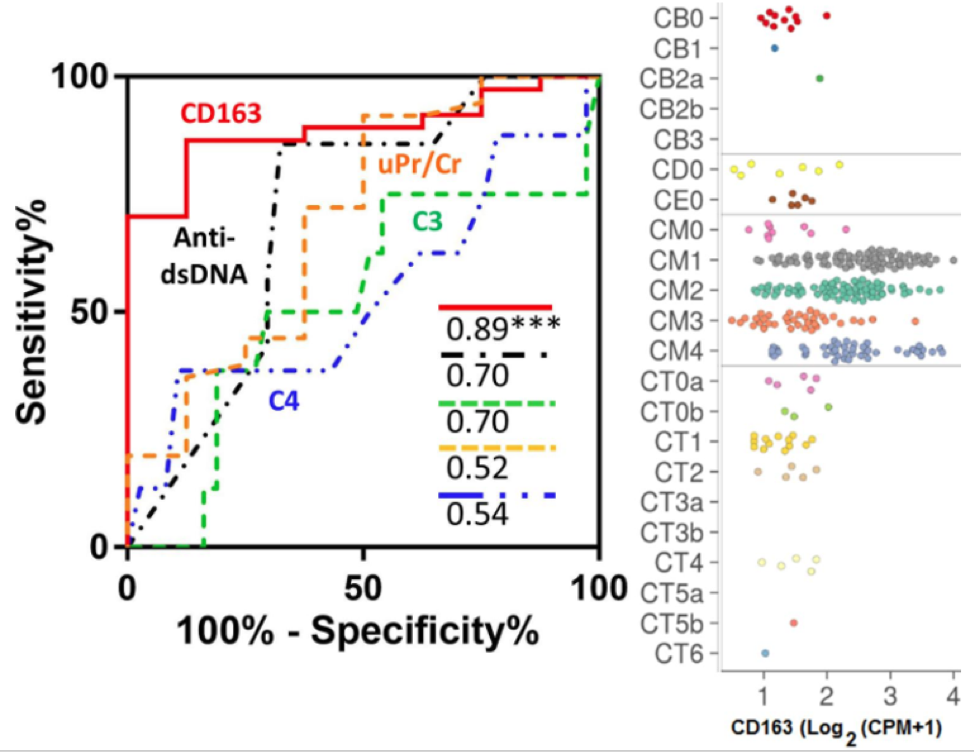
CD163 is a marker for alternatively activated macrophages, which have been implicated in the pathogenesis of lupus nephritis (LN). In our preliminary screening of urine proteins in LN, urine soluble CD163 (sCD163) was significantly elevated in patients with active LN. To evaluate the potential of sCD163 as a biomarker in LN, urine sCD163 was assayed in patients with active LN, active non-renal lupus patients (ANR), inactive SLE and healthy controls (HC), using ELISA and normalized to urine creatinine. A total of 228 SLE patients and 56 HC were included from three cohorts. Urine sCD163 was significantly elevated in active LN when compared with HC, inactive SLE, or ANR in African-American, Caucasian and Asian subjects (all P < 0.001). In LN patients with concurrent renal biopsies, urine sCD163 was significantly increased in patients with proliferative LN when compared with non-proliferative LN (P < 0.001). Urine sCD163 strongly correlated with SLEDAI, rSLEDAI, activity index (AI) of renal pathology, fibrinoid necrosis, cellular crescents, and interstitial inflammation on biopsies (all P < 0.01). Macrophages, particularly M2 macrophages, the predominant cells expressing CD163 within LN kidneys, represent a potential source of elevated urine sCD163, based on single-cell RNA sequence analysis of LN kidneys, as shown in the Figure.
What is already known in the field?
- Urinary biomarkers have now emerged as a potential tool for evaluating LN and potential treatment targets, as these proteins may arise directly from the inflamed kidneys. Several reported urine biomarkers including monocyte chemoattractant protein-1 (MCP-1), neutrophil gelatinase -B associated lipocalin (NGAL), TNF-like WEAK inducer of apoptosis (TWEAK), and vascular cell adhesion molecule-1 (VCAM), have been promising in monitoring disease status.
- We had previously reported that urine VCAM-1 and angiostatin can predict various pathological changes within LN kidneys.
What is new?
- Urine sCD163 was significantly elevated in active LN when compared with HC, inactive SLE, or ANR in African-American, Caucasian and Asian subjects.
- In LN patients with concurrent renal biopsies, urine sCD163 was significantly increased in patients with proliferative LN when compared with non-proliferative LN.
- Urine sCD163 strongly correlated with SLEDAI, rSLEDAI, AI of renal pathology, fibrinoid necrosis, cellular crescents, and interstitial inflammation on renal biopsies.
- Macrophages, particularly M2 macrophages, the predominant cells expressing CD163 within LN kidneys, represent a potential source of elevated urine sCD163, based on single-cell RNA sequence analysis of LN kidneys.
Why is this important?
- The identification of better biomarkers which significantly discriminate active LN and strongly correlate with renal biopsy findings or underlying disease mechanisms is of priority.
- Urine sCD163 discriminated patients with active LN from other SLE patients and was significantly elevated in proliferative LN. It strongly correlated with concurrent AI and several specific pathological attributes, demonstrating its potential in predicting concurrent renal pathology.
- Since the renal biopsy is invasive, urine sCD163 measurement, which is non-invasive, holds promise as a surrogate of renal pathology activity in patients with LN.
Ongoing/future steps:
- To validate urine sCD163 in longitudinal LN patient cohorts and evaluate whether it fluctuates concordantly with disease activity over serial follow-up
- To collect urine samples before biopsy in LN patients, and to confirm the ability of urine sCD163 to predict renal pathology
- To multiplex urine sCD163 with other urine markers to derive a biomarker panel that is predictive of the renal pathology activity index and chronicity index.