Identification of Low Abundance Biomarkers Using Electrochemiluminescence Assays
Lead: Dennis Tran
Team Members: Ting Zhang
Project Summary:
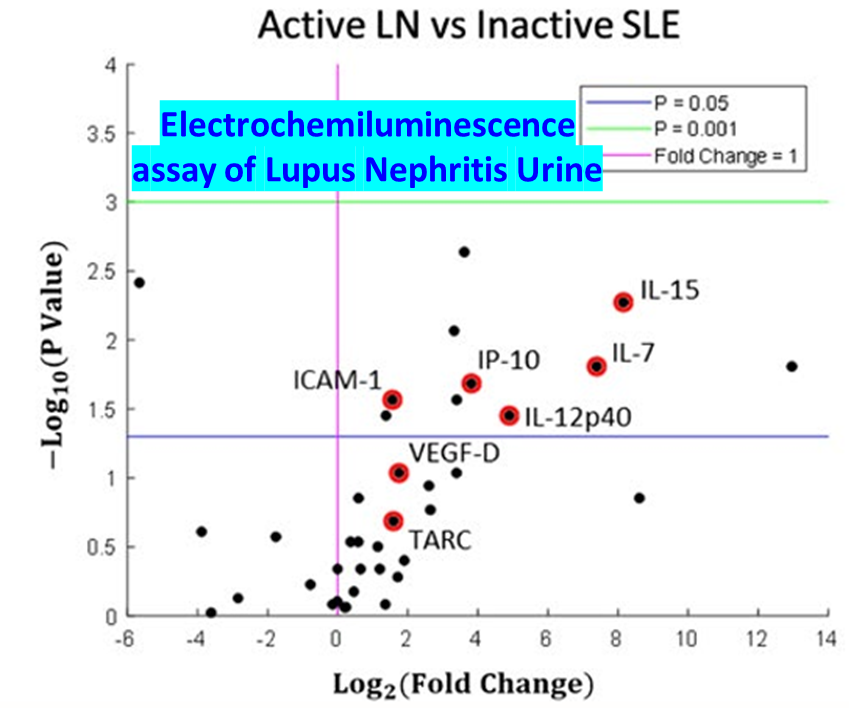
Systemic lupus erythematosus (SLE) is an autoimmune disease that progresses to chronic inflammation of the kidneys or lupus nephritis. Lupus nephritis (LN) is a leading cause of mortality and morbidity in SLE. A significant fraction of SLE patients will develop LN and some will develop end stage renal disease. The gold standard for renal monitoring in SLE is a renal biopsy. While renal biopsies are effective in providing prognostic information, they are also invasive and carry additional risks to the patient. As such, there is a need for better diagnostic techniques to monitor pathogenesis. Electrochemiluminescence (ECL) uses labels that allow for highly sensitive detection of autoantibodies/biomarkers in patient samples, with relatively simple and easy to use procedures. We have already shown that this highly sensitive platform can detect several urine biomarkers in LN that are otherwise missed by conventional assays such as ELISA (see picture). This project extends the use of this technology to interrogate additional biomarkers in the blood and urine of SLE/LN patients.
What is already known in the field?
- Early detection and diagnosis and earlier treatment has shown to be effective in LN.
- ECL uses labels called SULFO-TAG to increase sensitivity and reduce background, and also allows for better multiplexing.
- We have already shown that this highly sensitive platform can detect several urine biomarkers in LN that are otherwise missed by conventional assays such as ELISA (see picture).
What is new?
- The engineering of a 10-plex ultra-sensitive assay for detecting LN biomarkers
- The detection of novel biomarkers using ECL not detectable by ELISA, using urine samples from LN patients
Why is this important?
Renal biopsy is an invasive procedure that bears risks and can cause complications, along with not being able to be repeated often. This limits the monitoring and tracking of LN. A technique that is non-invasive, simple, easy-to-use, and capable of detecting small amounts of biomarkers from patient samples can facilitate better and more frequent monitoring of LN in SLE patients. This is important because earlier detection of renal involvement in SLE and prompt treatment can significantly improve outcome.
Ongoing/future steps:
- Validation and comparison of the ECL platform with the gold standard ELISA protocol for assaying selected biomarkers that may be clinically useful in diagnosing or monitoring patients with SLE, LN or other systemic rheumatic diseases.
- Engineer a 10-plex assay for assaying 10 low abundance biomarkers of interest in SLE/LN using ECL.