Spatial Proteomic and transcriptomic analysis of lupus nephritis underscore the association of tubular Galectin-3 with renal damage
Lead: Dr. Crosslee Titus, Ayesha Budhwani
Team Members: Ali Sherwani, Akanksha Pisati
Collaborators: Dr. Chen, Dr. Saxena, Dr. Truong
Project Summary:
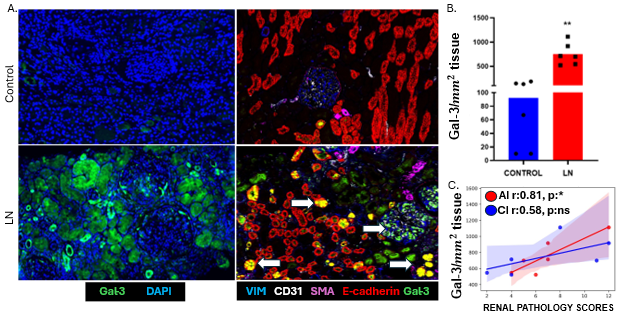
This project investigates the spatial distribution and functional role of Gal-3, a β-galactoside binding lectin, in the pathogenesis of Lupus Nephritis. By utilizing spatial proteomics and transcriptomics, the study aims to map Gal-3 expression within intact renal tissue to identify the specific cellular sources of Gal-3, specifically within renal and immune cell populations, to clarify its role in driving organ damage.
What is already known in the field?
- Gal-3 is well-documented as a pro-fibrotic mediator in various organs, including the heart and liver.
- In healthy mature kidneys, Gal-3 expression is typically restricted to distal tubules and specific collecting duct cells.
- It is known that Gal-3 levels are elevated in the serum of SLE patients, and some studies have noted its presence in SLE kidneys.
What is new?
- This study provides the first high-resolution characterization of Gal-3’s spatial coordinates within diseased kidneys relative to areas of tubular atrophy and fibrosis
Why is this important?
Gal-3 could serve as a high-precision biomarker for identifying patients at high risk for progressive fibrosis. By highlighting Gal-3 as a driver of disease progression rather than just a marker of damage, this research supports the development of Gal-3 inhibitors as a strategy to prevent End Stage Kidney Disease (ESKD) in LN.
Ongoing/future steps:
- Test whether targeted Gal-3 inhibition can stabilize the kidney microenvironment and reduce the recruitment of pro-inflammatory immune cells and subsequent renal damage in experimental models of lupus nephritis.