Aptamer based screen of >1000 proteins reveals novel stool markers of inflammatory bowel disease (IBD) that predict disease course
Lead: Kamala Vanarsa
Team: Malavika Nidhi, Ramya Susarla, Ting Zhang, Prashanth Sasidharan, Sanam Soomro, Suresh Venkateswaran
Collaborator: Drs. Kugathasan, Pedroza
Project Summary:
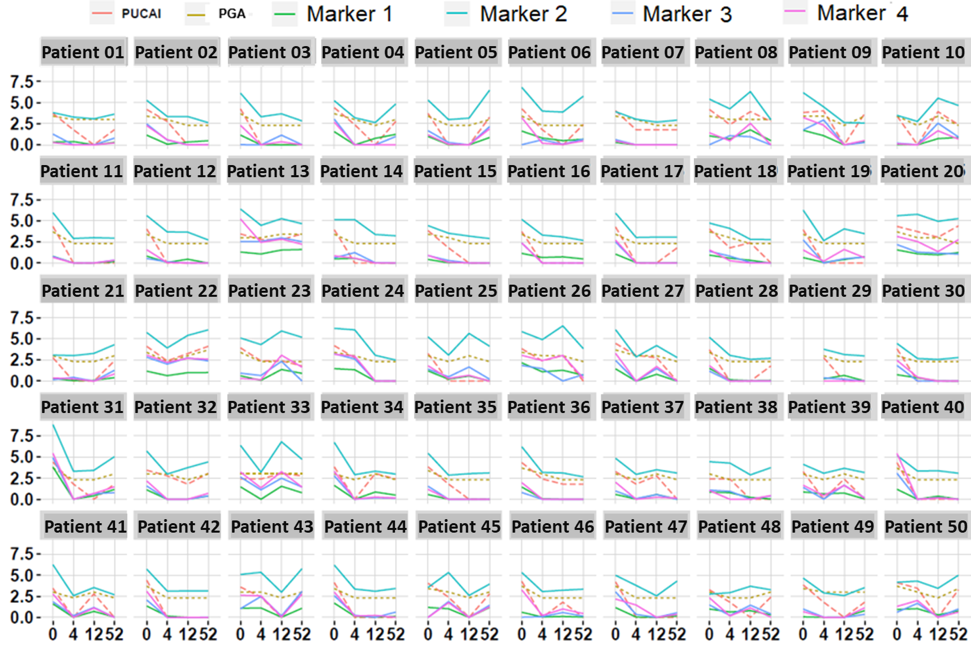
IBD affects ~1.6 million Americans, including as many as 80,000 children. Since IBD is a lifelong disease, often treated with intense immunosuppressive therapies, a firm diagnosis supported by endoscopically obtained tissue biopsies and histology is necessary for diagnosis. Since endoscopy is invasive, there is a need for non-invasive markers of clinical activity. Although serological testing appears promising in IBD stratification, stool biomarkers hold great promise as a non-invasive test, as stool is closer to the site of pathology and inflammation in IBD, and stool testing can be repeated as often as needed. With this goal in mind, we carried out an unbiased aptamer-based screen of 1129 stool proteins in IBD. This screen revealed several potential candidates and protein panels with superior predictive potential and clinical utility, both in cross-sectional and longitudinal cohorts of IBD patients. As shown in the figure, several of the identified stool proteins outperform current diagnostic tests in tracking disease activity over time, in a cohort of 50 IBD patients examined over 4 follow-up time points.
What is already known in the field?
- Close monitoring of disease and prompt treatment is beneficial in IBD.
- Fecal calprotectin is currently used as a biomarker in IBD, but is not optimal
- Stool tests have potential in monitoring gastrointestinal diseases, but are under-utilized.
What is new?
- Screening of >1000 stool proteins in IBD is novel.
- The platform used for proteomic screening, composed of a library of aptamers, is novel.
- The proteins uncovered using this novel platform are also novel, having superior diagnostic potential.
Why is this important?
- Since endoscopy is invasive, there is a need for non-invasive markers of clinical activity in IBD. Fecal calprotectin is currently used as a biomarker in IBD, but is sub-optimal. Hence there is an urgent need to identify non-invasive biomarkers with superior disease predictive potential.
- Since stool proteins may arise directly from intestinal lesions in IBD, they may shed light on disease pathogenesis mechanisms, and point to novel therapeutic targets for IBD.
Ongoing/future steps:
- Validation of these stool proteins in additional IBD cohorts, both in pediatric and adult Crohn’s Disease and Ulcerative Colitis.
- Randomized clinical trials using these stool biomarkers as indices to monitor treatment response are warranted.
- Applying larger screening platforms to identify and incorporate additional protein biomarkers with predictive potential in IBD
- Generation of easy to use point of care tests to monitor stool biomarkers in IBD