Gene – Gender Interplay in Lupus
Lead: A. R. Rajalakshmy
Team members: Yanping Chen, Rani Nune
Collaborators: Drs. Satterthwaite and Alarcon-Riquelme
Project Summary:
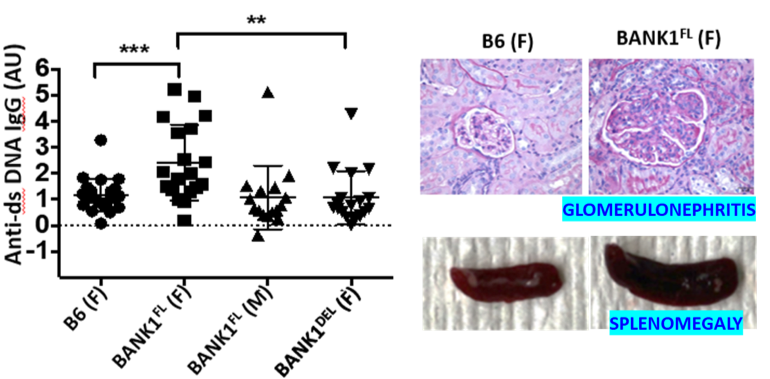
B-cell scaffold protein with ankyrin repeat 1 (BANK1) is expressed predominantly in B cells. GWAS studies and multiple meta-analysis studies have identified BANK1 as a disease gene for SLE and SSc. Although genetic studies suggest the association of BANK1 gene and SLE disease susceptibility, there have been no animal studies to explain the mechanistic link between human BANK1 gene and SLE. Human BANK1 protein is expressed as 2 variant isoforms. The full-length isoform is associated with SLE susceptibility and an exon 2 deleted isoform acts as a dominant negative and is not linked to SLE. To study the molecular mechanisms of human BANK1 mediated B cell immune alterations in SLE, we have developed a full length hBANK1Tg mice (BANK1 FLTg) model and an exon 2 deleted hBANK1Tg mice (BANK1 DELTg), both of which express the human versions of the gene. Preliminary studies show that only female mice bearing BANK1 FLTg develop lupus like disease. The overall goal of this project is to study B cell immune alterations and molecular mechanisms related to hBANK1 gene in SLE disease pathogenesis, and its interaction with gender.
What is already known in the field?
- BANK1 has been identified as a disease gene for SLE in multiple GWAS and meta-analysis studies.
- Alternative splicing of BANK1 leads to two isoforms. The full-length isoform is associated with SLE susceptibility and an exon 2 deleted isoform acts as a dominant negative and is not linked to SLE.
- BANK1 regulates autoantibody production in a lupus model.
What is new?
- Mechanistic studies to show that BANK1 isoforms regulate lupus differentially.
- Novel animal models expressing human BANK1 FL and BANK1 DEL isoforms
- Molecular mechanisms related to BANK1 and gender interplay in lupus
Why is this important?
The BANK1 gene is associated with SLE susceptibility. However, we do not know how this gene functions to cause lupus. Females are more prone to developing lupus, but the precise reasons for this are unclear. The novel transgenic model we have developed, where lupus develops predominantly in females as a consequence of just 1 disease gene, could help unravel how genes and gender interface to cause lupus.
Ongoing/future steps:
- To study the molecular mechanisms through which BANK1 synergize with female factors to promote lupus pathogenesis.
- To determine the mechanisms by which FL BANK1 affects B cell activation
- To assess if the BANK1 DEL can function as a dominant negative, with respect to its impact on lupus development using transgenic approaches.