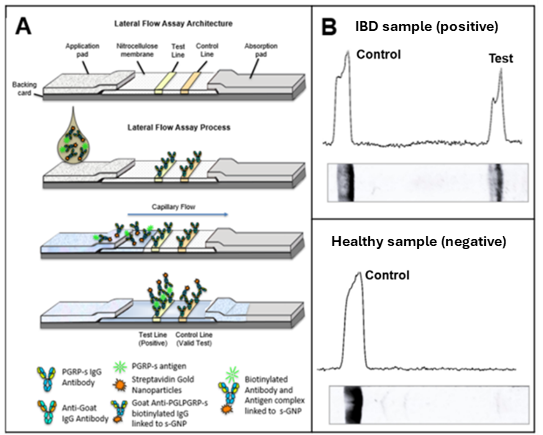
Engineering rapid lateral flow tests for monitoring stool PGRPS in Inflammatory Bowel Disease
Lead: Sonja Vodehnal
Team members: Anjumariya Kottarathil, Kimberly Hoang
Collaborators: Kannan Alpadi, Subra Kugathasan
Project Summary:
Inflammatory bowel diseases (IBD) are estimated to be affecting ~1.6 million Americans, and up to 70,000 new diagnoses each year – from young children to the elderly. Crohn’s disease (CD) and ulcerative colitis (UC) are the two main subphenotypes of IBD. Currently, endoscopy is the best test for diagnosis, but is invasive and costly. The annual mean health care cost for patients with IBD is over 3-fold higher than patients without IBD. Additionally, due to the immense preparation necessary, an endoscopy is challenging to repeat longitudinally. Our project’s main objective is to monitor a novel stool biomarker – Peptidoglycan recognition protein S (PGRP-s) – to quantitatively develop a point-of-care test for IBD, given that this stool marker has been shown to out-perform the current yardstick, fecal calprotectin. A more reliable and repeatable test for IBD could have a significant impact on early diagnosis, disease monitoring, and predicting/tracking response to treatment.
What is already known in the field?
- Fecal calprotectin, the current yardstick for IBD screening, distinguishes well between IBD and HC samples, but has significant limitations.
- Peptidoglycan recognition protein S (PGRPs) is know to be significantly upregulated in IBD stool, correlating with disease activity.
- Baseline stool PGRP-s can predict early clinical remission at week 4 (2021 Nature Communications 12: 3989), underscoring its potential for IBD monitoring.
What is new?
- The engineering of rapid IBD test strips designed for easy use, by patients, point of care, or reference labs
- Focusing on a novel and promising stool biomarker, PGRP-s.
Why is this important?
- Currently, endoscopy is the gold standard for diagnosing IBD and for assessing inflammation severity and location. However, this approach is invasive and costly.
- Fecal calprotectin, the current yardstick for IBD screening, distinguishes well between IBD and HC samples, but has significant limitations.
- Given the current limitations, the availability of an easy-to-use, inexpensive, reliable test for IBD could dramatically impact early diagnosis of IBD and subsequent patient management.
Ongoing/future steps:
- Perform multi-site collaborative studies to establish the utility, reliability and stability of the PGRP-s LFA test under field conditions
- Utilization of digital platform readers or cartridge-based assays for convenient signal detection
- Integration of additional biomarkers to create a multiplex LFA that would supplement PGRP-s for subphenotypic differentiation.