Pan-Proteome Screening for Novel Autoantibodies in Lupus Nephritis
Lead: Manisha Madhusudan
Team Members: Akanksha Pisati, Vineetha Chidipoth, Kamala Vanarsa, Yewei Ma
Project Summary:
Systemic lupus erythematosus (SLE) is an autoimmune disease driven by pathogenic autoantibodies that cause inflammation and organ damage. Lupus nephritis (LN), the renal manifestation of SLE, affects up to 50% of patients and remains a major cause of kidney failure. Despite this, current clinical tests assess only a limited number of known autoantibodies and fail to capture the full immune complexity of LN.
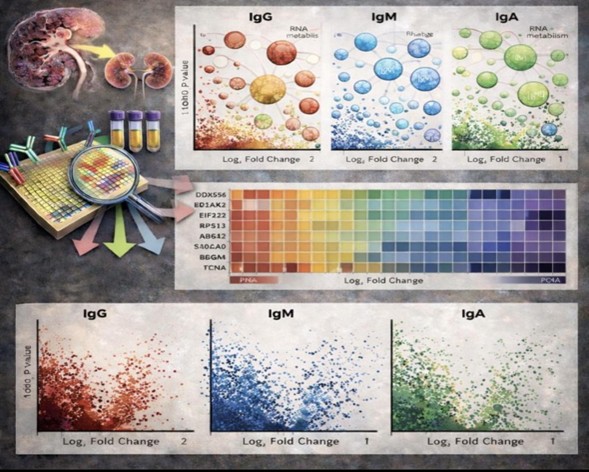
In this project, we use pan-proteome autoantibody profiling to identify both known and novel autoantibody targets in lupus nephritis. Using a high-density human proteome array, we simultaneously profile IgG, IgA, and IgM autoantibodies in LN patients and controls. Pathway-level analysis is then used to identify biological processes preferentially targeted by lupus autoantibodies.
What is already known in the field?
Autoantibodies are central to SLE and lupus nephritis pathogenesis, yet existing biomarkers capture only a fraction of disease-associated immune responses. Autoantibody profiles vary substantially between patients and may reflect distinct disease mechanisms.
What is new?
This study applies an unbiased, proteome-wide approach to define the autoantibody landscape in LN. We have now identified previously unrecognized IgG, IgA, and IgG autoantigens and show that many targets are involved in RNA metabolism, translation, and cellular regulatory processes.
Why is this important?
By expanding the known autoantigen repertoire and linking novel targets to biologically meaningful pathways, this work provides new insight into LN pathogenesis. These findings may inform the development of improved biomarkers and support more precise disease monitoring and therapeutic strategies.
Ongoing/Future Steps
Ongoing work focuses on validating novel autoantibody targets, correlating antibody profiles with clinical features, and extending these analyses to longitudinal samples to assess changes over time.