Spatial Transcriptomics of Hypoxia and Immune-Mediated Damage in Lupus Nephritis
Leads: Aalekhya Biswas, Anto Sam Crosslee Titus
Collaborators: Vishal Srinivasan , Vishal Surya , Rohith Appalaneni , Shu-Hsia Chen, Ramesh Saxena, Qi Cai, Luan Truong,
Project Summary:
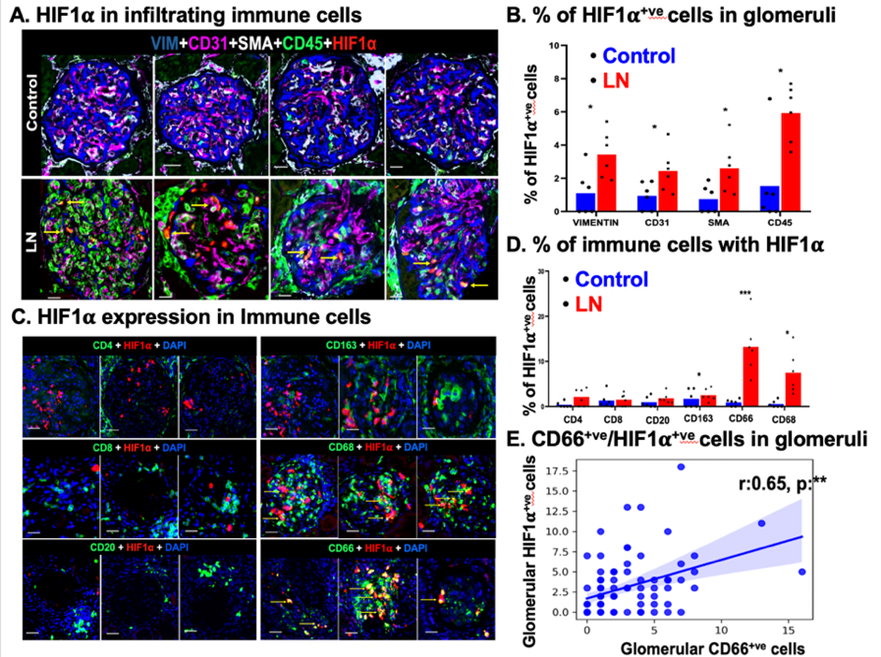
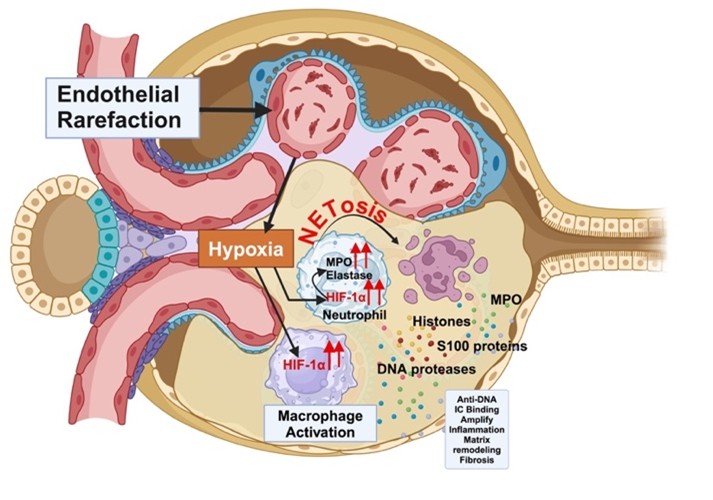
Lupus nephritis (LN) is a severe manifestation of systemic lupus erythematosus characterized by immune-mediated glomerular injury, progressive renal hypoxia, and loss of microvascular integrity. In this study, we investigated the spatial and molecular interplay between hypoxia and immune-mediated damage in renal tissue from patients with lupus nephritis. Renal biopsy samples were analyzed using multiplexed cyclic immunofluorescence and spatial transcriptomics. LN glomeruli demonstrated a marked accumulation of hypoxia-inducible factor–1α (HIF1α)–positive macrophages and neutrophils. Neutrophils exhibited prominent perinuclear myeloperoxidase (MPO) staining, consistent with active neutrophil extracellular trap (NET) formation. Spatial transcriptomic profiling further confirmed a strong association between hypoxia-related gene signatures and NETosis pathways within affected glomeruli. Importantly, glomerular endothelial cell rarefaction strongly correlated with increased numbers of hypoxic neutrophils and a reduction in capillary network complexity. Complementing these tissue-based findings, urinary analysis revealed elevated levels of neutrophil-derived cargo proteins, including MPO and proteinase 3, suggesting their potential utility as noninvasive liquid biopsy biomarkers of renal pathology and disease activity. Collectively, these findings provide new mechanistic insight into LN pathogenesis, highlighting how endothelial injury–driven hypoxia shapes pathogenic myeloid cell phenotypes that promote ongoing inflammation, scarring, and renal dysfunction.
What Is Already Known on This Topic?
- Lupus nephritis results from immune complex deposition within the kidney, triggering complement activation, inflammation, and progressive glomerular damage.
- Renal hypoxia is a recognized feature of LN and is thought to exacerbate inflammation and fibrosis by altering immune cell function and endothelial integrity.
- Neutrophils and other myeloid cells contribute significantly to LN progression through activation, cytokine release, and the formation of neutrophil extracellular traps (NETs), which amplify tissue injury and autoimmunity.
What This Study Adds?
- This study identifies glomerular endothelial cell rarefaction as a key determinant of sustained renal hypoxia in lupus nephritis. It demonstrates that hypoxic neutrophils are enriched within LN glomeruli and are major contributors to tissue damage through enhanced NETosis.
- The findings suggest that reparative or N2-like neutrophil phenotypes, while initially adaptive, may paradoxically promote fibrosis, scarring, and progressive kidney injury in LN. Spatial transcriptomics provides direct evidence linking hypoxia-responsive gene programs to neutrophil activation and NET formation in situ.
Future steps:
- Measurement of urinary neutrophil cargo proteins and NET-associated markers (such as MPO and proteinase 3) could serve as a noninvasive liquid biopsy to assess renal hypoxia, immune activation, and pathological activity in LN patients.
- Therapeutic strategies aimed at preserving or restoring glomerular endothelial integrity may reduce hypoxia-driven immune injury and slow disease progression.
- Targeting hypoxia-responsive pathways or pathogenic neutrophil phenotypes represents a promising avenue for adjunctive therapies beyond conventional immunosuppression.