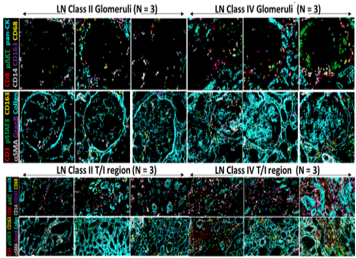
35-plex CyTOF staining of lupus nephritis kidneys to identify novel predictors of disease severity
Lead: Crosslee Titus
Team members: Pietro Cicalese
Collaborator: Dr. S-H Chen
Project Summary:
Lupus Nephritis (LN) is one of the leading causes of morbidity and mortality in Systemic Lupus Erythematosus and 60% of lupus patients develop LN. LN leads to chronic kidney disease (CKD) and End-Stage Renal Disease (ESRD). Currently, there is no cure for LN and current medications are either not molecularly targeted or have serious side-effects. Treatments are based on the symptoms and severity of the disease and are timed with disease flares. Renal biopsies are used for LN classification, but is subject to observer bias. Currently, most of the information obtained from the biopsy is derived from generic stains used to highlight tissue morphology, sometimes supplemented with limited immunohistochemistry. The inability to multiplex using conventional IHC/IF has hampered the identification of molecular predictors of disease progression and pathogenic pathways in LN.
What is already known in the field?
- Current LN classification is based on tissue morphology but is subject to observer bias, and not driven by the underlying molecular basis of the disease.
- Tissue CyTOF or imaging mass cytometry has made multiplexed analysis of tissues possible, unlike IHC/IF.
What is new?
- Compared to conventional immunohistochemistry (or IF) which relies on flurophores / enzymes conjugated to antibodies (limited by species compatibility and fluorescent spectrum), imaging mass cytometry (IMC) uses antibodies which are conjugated to metal tags.
- This approach allows 35 or more antibodies to be multiplexed simultaneously for staining. Next, a Nd:YAG 213 nm laser is used to ablate 1um2 areas of tissue at a time which are then analyzed using the Fluidigm CyTOF mass cytometer.
- This approach preserves tissue architecture and spatial information while still providing molecule expression data effectively at a single cell level. This enable the elucidation of cellular and molecular pathways underling disease in any given tissue.
Why is this important?
- Compared to morphological classification of LN, classification based on molecular pathways will yield a richer perspective of the disease because judicious choice of the 35 or more staining antibodies can shed information on the role of a wide spectrum of molecular pathways and cell types in disease.
- Determining the expression profile of multiple molecules (35 or more) at a cellular level in the context of the tissue architecture can help better classify LN based on molecular expression, understand the nuances underlying disease heterogeneity in LN and help guide customized, targeted and individualized treatments.
- Tissue CyTOF of inflamed kidneys will pave the path towards personalized medicine in LN.
Ongoing/future steps:
- Expand the number of molecules interrogated on each LN kidney from 35 to 100
- Identify the molecular pathways which are critical at each stage of LN disease progression, as this will enable the development of targeted therapeutics at each stage
- Correlate the expression levels of renal biopsy biomarkers with the corresponding urinary and serum biomarkers to enable easy screening and surveillance of LN disease progression.
- To determine how various molecular signatures in LN kidneys predict concurrent clinical disease, as well as long term outcome in LN.