The 1000-plex antibody array platform as a discovery tool for identifying novel biomarkers of renal lupus
Lead: Kamala Vanarsa
Team Members: Sanam Soomro, Ting Zhang, Briony Strachan, Malavika Nidhi, Pietro Cicalese, Christopher Gidley, Shobha Dasari, Shree Mohan, Nathan Thai
Collaborator: Drs. Saxena, Putterman, Petri, Pedroza
Project Summary:
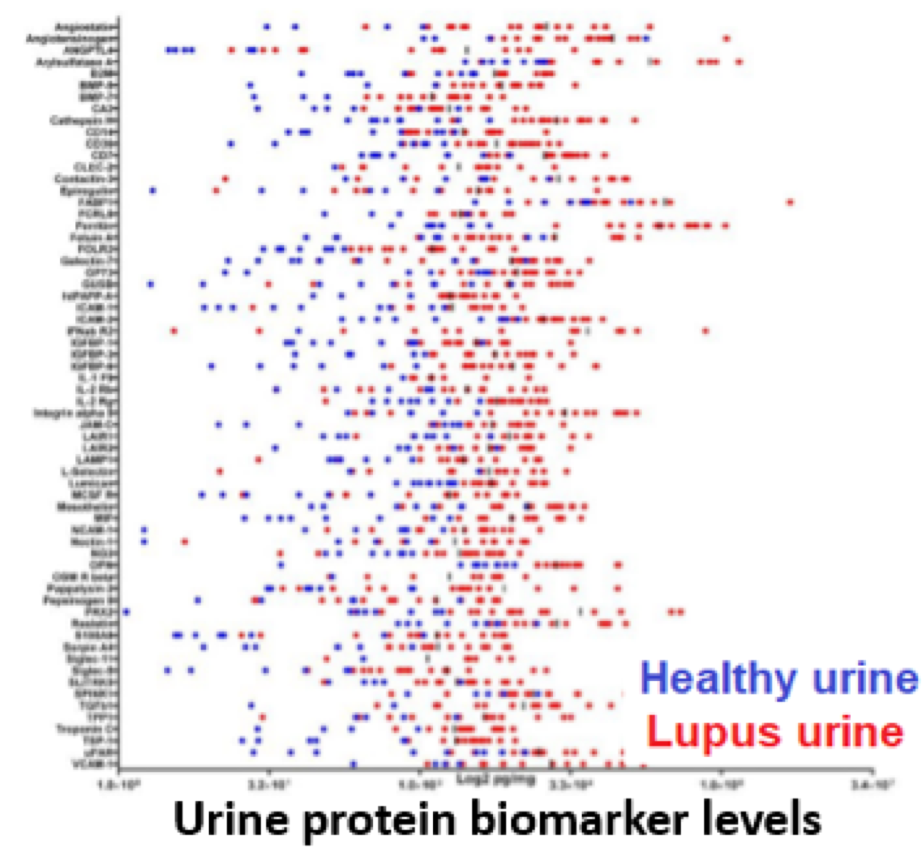
To discover novel urinary biomarkers of lupus nephritis (LN), urine from systemic lupus erythematosus (SLE) patients were interrogated for 1000 proteins using a novel, quantitative planar protein microarray. Hits were validated in an independent SLE cohort with inactive, active non-renal (ANR), and active renal (AR) patients, in a cohort with concurrent renal biopsies, and in a longitudinal cohort. Screening of 1000 proteins revealed 64 proteins to be significantly elevated in SLE urine, of which 17 were ELISA validated in independent cohorts. Urine Angptl4 (AUC=0.96), L-selectin (AUC=0.86), TPP1 (AUC=0.84), TGFb1 (AUC=0.78), TSP-1 (AUC=0.73), FOLR2 (AUC=0.72), PDGFRb (AUC=0.67), and PRX2 (AUC=0.65), distinguished active renal from ANR SLE, outperforming anti-DNA, C3 and C4, in terms of specificity, sensitivity, and PPV. In multivariate regression analysis, urine Angptl4, L-selectin, TPP1 and TGFb1 were highly associated with disease activity, even after correction for demographic variables. In SLE patients with serial follow-up, urine L-selectin (followed by urine Angptl4 and TGFb1) were best at tracking concurrent or pending disease flares. Importantly, several proteins elevated in LN urine were also expressed within the kidneys in LN, either within resident renal cells or infiltrating immune cells, based on single-cell RNA-sequence analysis.
What is already known in the field?
- Most methods utilized previously for biomarker detection have adopted a biased approach, based on exploring established pathophysiological pathways (or biomolecules) associated with SLE. While useful, these approaches limit the discovery of novel biomarkers and their associated pathways.
- As opposed to previous urine biomarker studies in lupus nephritis (LN), a comprehensive, unbiased, affinity-based, quantitative screen of 1000 specific proteins has been conducted using urine from patients with LN in this study.
What is new?
- Identification of urine Angptl4, L-selectin, TPP1, transforming growth factor-b1 (TGFb1), thrombospondin-1 (TSP-1), FOLR2, platelet-derived growth factor receptor-b (PDGF-Rb) and PRX2 as novel biomarkers of LN.
- Urine Angptl4, L-selectin, TPP1, TGFb1, TSP-1, FOLR2 and PDGF-Rb successfully distinguished active LN patients from active non-renal lupus patients, despite both groups having comparable SLEDAI scores.
- Urine Angptl4, L-selectin and TPP1, in combination, offered the best discrimination of active LN from active non-renal lupus, with an area under the receiver operating curves of 0.97.
Why is this important?
- Having more accurate biomarkers for predicting renal involvement in patients with lupus can facilitate early detection of disease in lupus. This is important because early treatment of renal lupus is associated with better outcome.
- Urine is easy to obtain and urine biomarkers can be serially tracked, even by the patient from home, using a POC assay format.
Ongoing/future steps:
- Validate the performance of these biomarkers in additional ethnic groups and larger patient cohorts.
- Validate these biomarkers in concurrent renal biopsy samples (with renal pathology data).
- Expand longitudinal study to investigate how these urinary molecules relate to renal pathology, disease progression, treatment response over time and long-term renal and patient outcome.
- Perform mechanistic studies to confirm the cellular origins of the identified biomarkers and to dissect out their respective roles in disease pathogenesis.